Pilot Study Report

Two separate randomized controlled studies were conducted: one only for diagnosed OSAS (Obstructive Sleep Apnea Syndrome), the other for snoring (Rhonchopathie) and UARS (Upper Airway Resistance Syndrome). Each study included 95 participants. The total dropout rate was less than 10%. The observation period for both studies was 3 months starting with April 2023.
For measuring the outcome, two standard questionnaires from sleep medicine were taken to assess various aspects of sleep quality and daytime sleepiness: the Pittsburgh Sleep Quality Index (PSQI) and the Epworth Sleepiness Scale (ESS).
Rhonchopathie/UARS
OSAS
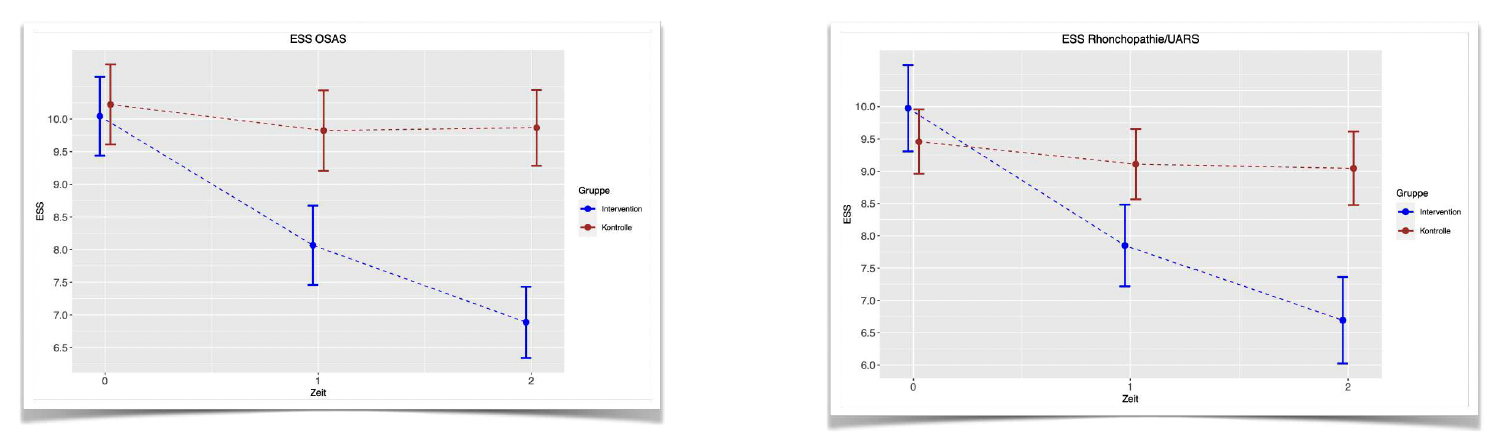
At time 0, the mean scores of ESS of the control groups in both studies (OSAS and Rhonchopathie / UARS) are almost equal to the mean ESS score in the intervention groups. Throughout the study, the mean ESS scores in the control groups remained almost the same at time 2. The mean ESS scores in the intervention groups improved significantly till time 2.
This difference between control groups and intervention groups is statistically significant.
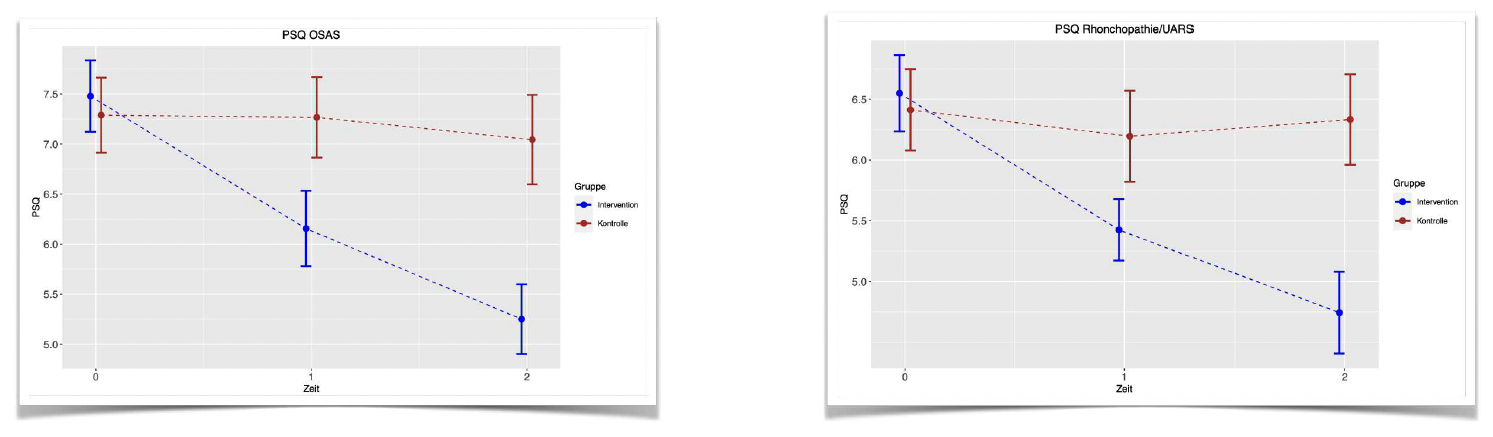
At time 0, the mean scores of PSQI of the control groups in both studies (OSAS and Rhonchopathie / UARS) are almost equal to the mean ESS score in the intervention groups. Throughout the study, the mean ESS scores in the control groups remained almost the same at time 2. The mean ESS scores in the intervention groups improved significantly till time 2.
This difference between control groups and intervention groups is statistically significant.